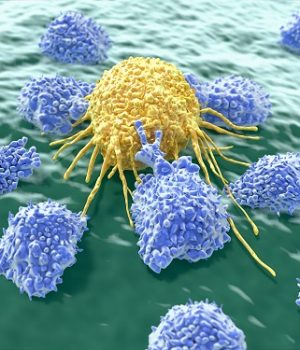
CAR T-cell therapy is not yet approved by the FDA and is considered investigational; a patient must enter a clinical trial to receive it. Here’s the process:
- Leukapheresis: The patient’s T-cells are harvested through apheresis and separated from other blood components such as red blood cell, platelets and monocytes. The amounts of specific subsets of T cells, such as CD4 and CD8 may be optimized; however, the optimal subsets of T-cells to improve efficacy and limit toxic effects are still under study.
- T-cell activation: T-cell activation occurs via primary and costimulatory signals. Endogenous cell-based activation, such as with dendritic cells, may be used, but is not reliable and requires further study. In beads-based activation, antibody-coated beads serve as artificial dendritic cells and can activate the isolated T cells. Biotechnology companies have available these established and accessible activators.
- Transduction: In the lab, the activated T-cells are genetically reprogrammed (DNA encoding) using lentiviral or gamma-retroviral recombinant vectors or a transposon system to yield the CAR construct receptor on the surface of the T-cell.
- Expansion of CAR T-cells: There are several CAR T-cell expansion methods that can be used in the lab to ensure a therapeutic dose of genetically modifed cells can be delivered to the patient. Once reproduced, the cells are frozen to ensure stability prior to delivery to the patient for infusion.
On average, the engineering process takes about 2 weeks. This lengthy timeline can be problematic for patients with rapidly progressive disease.